'Honeycomb' electrode makes redox flow battery more efficient
The pore size can easily be tuned to meet the desired application.

A relatively unknown type of battery – the redox-flow battery – is very promising for large-scale energy storage. To improve the electrochemical reactions in this battery, a team of researchers from Eindhoven University of Technology (������ý), DIFFER and MIT developed a completely new electrode with ‘honeycomb’ pores. With this material, the battery becomes more efficient and can be tailored to many different applications. Moreover, the new electrode is easier and cheaper to produce and suits large-scale manufacturing. The research results are published in the journal Advanced Materials.
Energy storage is one of the biggest hurdles for the energy transition. Renewable energy sources, like solar and wind energy, are not always available when we need them. They are not predictable, let alone adjustable. And our energy system is designed in such a way that the generated energy must be used immediately. Batteries are expected to be a big part of the solution; they store electrical energy as chemical energy so that sufficient power is available during times of high demand.
All batteries work on redox reactions; molecules that exchange electrons. The reductant loses an electron after which the oxidant absorbs an electron. When separated, which is the case in a battery, the resulting charge difference creates a current of electrons: electricity. A battery consists roughly of four parts: electrodes on both sides that initiate the redox-reaction, an electrolyte with the reductant and oxidant (positive and negative poles) where the reaction takes place, a membrane to keep the two electrolytes separate and an external electrical circuit.
Flow battery: cheap, scalable and safe
So far, lithium-ion batteries are the most widely-deployed type of battery. They can be found in mobile phones, laptops and in electric cars. These batteries are particularly suitable for domestic use and in the transport sector: small, lightweight and with a high energy density. Big disadvantages: they are quite expensive, highly flammable, use rare materials, are not easily scalable and they get exhausted over time.
For large-scale storage, in the order of hundreds of megawatts, this battery type is therefore not suitable. In those circumstances size and weight of the battery matter less than price, scalability and safety. It is here that an alternative type of battery, called the redox-flow battery, shows much promise.
NASA developed the redox-flow battery over 50 years ago. Until now, its further development has stalled because lithium-ion batteries were more useful for current applications due to their smaller size and weight. But now that entire cities are desperate for storage capacity, this alternative is suddenly drawing attention.
Independent of power and capacity
The redox-flow battery is called after the liquid electrolyte solution that flows through the reactor and is stored in separate tanks: the solution in one tank is negatively charged and the solution in the other tank is positively charged.
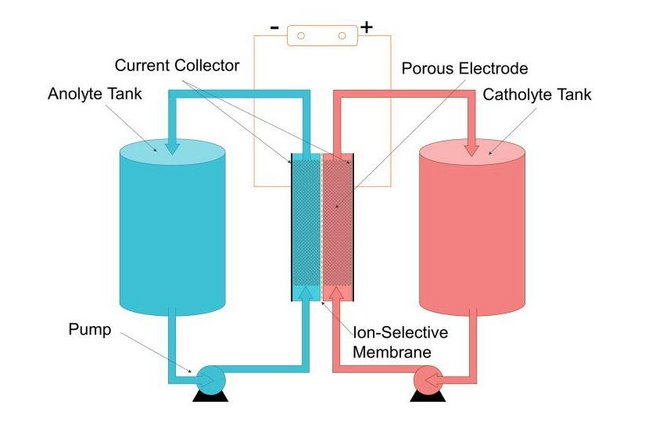
Contrary to the lithium-ion variant, redox-flow batteries can increase their capacity (kWh) without increasing their power (kW), and vice versa. By connecting a bigger tank with electrolyte solution to the system, you can increase the capacity in a rather simple way.
Redox-flow batteries become cost competitive when used at long discharge durations. In addition, redox-flow batteries are much safer than lithium-ion batteries since the components are liquid and not flammable. Redox-flow batteries are also more durable due to the lack of so-called intercalation reactions that negatively affect the stability of sealed batteries like lithium-ion batteries.
While redox-flow batteries are very promising, the current costs are still too high for widespread deployment. Further development is needed to bring down costs and improve efficiency and production. Researchers from ������ý and DIFFER have been working together with MIT scientists to improve the design in order to increase the efficiency of this battery type.
Mutually exclusive?
Batteries generate most power when the electrons flow fast. The electrode of a redox-flow battery therefore has a porous structure. Lead researcher Antoni Forner-Cuenca from ������ý: “The larger the pores, the easier the electrolyte can flow through and the lower the pressure loss. However, if the pores are too large, we renounce surface area. In an ideal situation, you would like both a high flow rate and a large reaction surface.”
At this moment, redox-flow batteries use conventional carbon fiber electrodes, the electrode type designed for low temperature fuel cells. However, these electrodes are complex and expensive to produce, and the production process makes the three-dimensional structure of the pores difficult to adjust to the desired application.
Membrane science provided the solution
Forner-Cuenca continues: “To develop a better performing electrode, we went back to the drawing table. We completely reconsidered and improved the design and choice of materials. Drawing inspiration from membrane science and technology, we used polymer phase separation to control the electrode structure.”
“We start with a liquid solution that contains two polymers. After immersion in water, a porous structure is formed by dissolving one of the polymers, the so called pore forming agent. We can play with the composition, solvent, temperature and other parameters to precisely control the porous structure of the electrode. It was those insights that formed the basis for our new design,” explains Forner Cuenca.
After numerous computer simulations and experimental work, the researchers managed to develop a material in which the pore size and shape of the electrode can be easily adjusted by varying the amounts of solvent and polymers. Forner-Cuenca: “The manufacturing process of our new material is much simpler and cheaper, offers larger versatility and is easier to upscale than the conventional electrode production. It shows that it is indeed possible to create an electrode with both favorable bulk electron transport and a large reaction surface.”
‘Honeycomb’ structure increases efficiency
One of the developed structures proved to be a hit: the 'honeycomb' electrode shows a promising combination of large and small pores. It therefore suits the application of large-scale energy storage. The large pores guarantee the high flow rate, as if it were a highway. And the small pores in between ensure sufficient reaction surface, being the ‘regional roads’.
Forner-Cuenca: “The experimental work was carried out by PhD-candidates Charles Tai-Chieh Wan from MIT and Remy Jacquemond from ������ý and DIFFER. They both separately managed to repeat the process in the labs at ������ý and MIT and therefore have proven reproducibility of the material. We are currently working on optimizing durability and scaling up the process. These initial results are very promising and we are excited to explore other applications for our electrodes.”
This research was published at March 2nd in the journal Advanced Materials. Title: Nonsolvent-Induced Phase Separation Enables Designer Redox Flow Battery Electrodes. DOI:
Media contact
Latest news
![[Translate to English:] Foto: Bart van Overbeeke Bewerking: Grefo](https://assets.w3.tue.nl/w/fileadmin/_processed_/f/7/csm_hoofdbeeld_def_c49a59b323.jpg)

